Pyrite
| Pyrite | |
|---|---|
A mass of intergrown pyrite crystals |
|
| General | |
| Category | Sulfide mineral |
| Chemical formula | iron disulfide (FeS2) |
| Strunz classification | 02.EB.05a |
| Dana classification | 2.12.1.1 |
| Crystal symmetry | Isometric diploidal Space group: Pa3 H-M symbol: 2/m3 |
| Unit cell | a = 5.417 Å, Z=4 |
| Identification | |
| Color | Pale brass-yellow, tarnishes darker and iridescent |
| Crystal habit | Cubic, faces may be striated, but also frequently octahedral and pyritohedron. Often inter-grown, massive, radiated, granular, globular and stalactitic. |
| Crystal system | Isometric |
| Twinning | Penetration and contact twinning |
| Cleavage | Indistinct on {001}; partings on {011} and {111} |
| Fracture | Very uneven, sometimes conchoidal |
| Tenacity | Brittle |
| Mohs scale hardness | 6–6.5 |
| Luster | Metallic, glistening |
| Streak | Greenish-black to brownish-black; smells of sulfur |
| Diaphaneity | Opaque |
| Specific gravity | 4.95–5.10 |
| Fusibility | 2.5–3 to a magnetic globule |
| Solubility | Insoluble in water |
| Other characteristics | paramagnetic |
| References | [1][2][3][4] |
The mineral pyrite, or iron pyrite, is an iron sulfide with the formula FeS2. This mineral's metallic luster and pale-to-normal, brass-yellow hue have earned it the nickname fool's gold because of its resemblance to gold. The color has also led to the nicknames brass, brazzle and Brazil, primarily used to refer to pyrite found in coal.[5][6]
Pyrite is the most common of the sulfide minerals. The name pyrite is derived from the Greek πυρίτης (puritēs), "of fire" or "in fire",[7] in turn from πύρ (pur), "fire".[8] In ancient Roman times, this name was applied to several types of stone that would create sparks when struck against steel; Pliny the Elder described one of them as being brassy, almost certainly a reference to what we now call pyrite.[9] By Georgius Agricola's time, the term had become a generic term for all of the sulfide minerals.[10]
Pyrite is usually found associated with other sulfides or oxides in quartz veins, sedimentary rock, and metamorphic rock, as well as in coal beds, and as a replacement mineral in fossils. Despite being nicknamed fool's gold, pyrite is sometimes found in association with small quantities of gold. Gold and arsenic occur as a coupled substitution in the pyrite structure. In the Carlin, Nevada gold deposit, arsenian pyrite contains up to 0.37 wt% gold.[11]
Contents |
Weathering and release of sulfate
Pyrite exposed to the atmosphere during mining and excavation reacts with oxygen and water to form sulfate, resulting in acid mine drainage. This acidity results from the action of Acidithiobacillus bacteria, which generate their energy by oxidizing ferrous iron (Fe2+) to ferric iron (Fe3+) using oxygen. The ferric iron in turn attacks the pyrite to produce ferrous iron and sulfate. The ferrous iron is then available for oxidation by the bacterium; this cycle continues until the pyrite is depleted.
Iron pyrite oxidation is sufficiently exothermic that underground coal mines in high-sulfur coal seams have occasionally had serious problems with spontaneous combustion in the mined-out areas of the mine. The solution is to hermetically seal the mined-out areas to exclude oxygen.[12]
In modern coal mines, limestone dust is sprayed onto the exposed coal surfaces to reduce the hazard of dust explosions. This has the secondary benefit of neutralizing the acid released by pyrite oxidation and therefore slowing the oxidation cycle described above, thus reducing the likelihood of spontaneous combustion. In the long term, however, oxidation continues, and the hydrated sulfates formed may exert crystallization pressure that can expand cracks in the rock and lead eventually to roof fall.[13]
Building stone containing pyrite tends to stain brown as the pyrite oxidizes. This problem appears to be significantly worse if any marcasite is also present.[14] The presence of pyrite in the aggregate used to make concrete can lead to severe deterioration as the pyrite oxidizes.[15] In early 2009, problems with Chinese drywall imported into the United States after Hurricane Katrina were attributed to oxidation of pyrite.[16]
Uses
Pyrite enjoyed brief popularity in the 16th and 17th centuries as a source of ignition in early firearms, most notably the wheellock, where the cock held a lump of pyrite against a circular file to strike the sparks needed to fire the gun.
Pyrite has been used since classical times to manufacture copperas, or iron sulfate. Iron pyrite was heaped up and allowed to weather as described above (an early form of heap leaching). The acidic runoff from the heap was then boiled with iron to produce iron sulfate. In the 15th century, such leaching began to replace the burning of sulfur as a source of Sulfuric Acid. By the 19th century, it had become the dominant method. [17]
Pyrite remains in commercial use for the production of sulfur dioxide, for use in such applications as the paper industry, and in the manufacture of sulfuric acid. Thermal decomposition of pyrite into FeS (iron sulfide) and elemental sulfur starts at 550 °C; at around 700 °C pS2 is about 1 atm.[18]
Pyrite is a semiconductor material with band gap of 0.95 eV.[19]
During the early years of the 20th century, pyrite was used as a mineral detector in radio receivers, and is still used by 'crystal radio' hobbyists. Until the vacuum tube matured, the crystal detector was the most sensitive and dependable detector available- with considerable variation between mineral types and even individual samples within a particular type of mineral. Pyrite detectors occupied a midway point between galena detectors and the more mechanically complicated perikon mineral pairs. Pyrite detectors can be as sensitive as a modern 1N34A diode detector.[20][21]
Pyrite has been proposed as an abundant, inexpensive material in low cost photovoltaic solar panels.[22] Synthetic iron sulfide is used with copper sulfide to create the experimental photovoltaic material.[23]
Pyrite is used to make marcasite jewelry (incorrectly termed marcasite). Marcasite jewelry, made from small faceted pieces of pyrite, often set in silver, was popular in the Victorian era.[24]
Formal oxidation states for pyrite, marcasite, and arsenopyrite
From the perspective of classical inorganic chemistry, which assigns formal oxidation states to each atom, pyrite is probably best described as Fe2+S22−. This formalism recognizes that the sulfur atoms in pyrite occur in pairs with clear S–S bonds. These persulfide units can be viewed as derived from hydrogen disulfide, H2S2. Thus pyrite would be more descriptively called iron persulfide, not iron disulfide. In contrast, molybdenite, MoS2, features isolated sulfide (S2−) centers. Consequently, the oxidation state of molybdenum is Mo4+. The mineral arsenopyrite has the formula FeAsS. Whereas pyrite has S2 subunits, arsenopyrite has AsS units, formally derived from deprotonation of H2AsSH. Analysis of classical oxidation states would recommend the description of arsenopyrite as Fe3+(AsS)3−.[25]
Crystallography
Iron-pyrite FeS2 represents the prototype compound of the crystallographic pyrite structure. The structure is simple cubic and was among the first crystal structures solved by X-ray diffraction.[26] It belongs to the crystallographic space group Pa3 and is denoted by the Strukturbericht notation C2. Under thermodynamic standard conditions the lattice constant 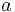 of stoichiometric iron pyrite FeS2 amounts to 541.87 pm.[27] The unit cell is composed of a Fe face-centered cubic sublattice into which the S ions are embedded. The pyrite structure is also taken by other compounds MX2 of transition metals M and chalcogens X = O, S, Se and Te. Also certain dipnictides with X standing for P, As and Sb etc. are known to adopt the pyrite structure.[28]
of stoichiometric iron pyrite FeS2 amounts to 541.87 pm.[27] The unit cell is composed of a Fe face-centered cubic sublattice into which the S ions are embedded. The pyrite structure is also taken by other compounds MX2 of transition metals M and chalcogens X = O, S, Se and Te. Also certain dipnictides with X standing for P, As and Sb etc. are known to adopt the pyrite structure.[28]
In the first bonding sphere, the Fe atoms are surrounded by six S nearest neighbours, in a distorted octahedral arrangement. The material is a diamagnetic semiconductor and the Fe ions should be considered to be in a low spin divalent state (as shown by Mösbauer spectroscopy as well as XPS), rather than a tetravalent state as the stoichiometry would suggest.
The positions of X ions in the pyrite structure may be derived from the fluorite structure, starting from a hypothetical Fe2+(S-)2 structure. Whereas F- ions in CaF2 occupy the centre positions of the eight subcubes of the cubic unit cell (¼ ¼ ¼) etc., the S- ions in FeS2 are shifted from these high symmetry positions along <111> axes to reside on (uuu) and symmetry-equivalent positions. Here, the parameter u should be regarded as a free atomic parameter that takes different values in different pyrite-structure compounds (iron pyrite FeS2: u(S) = 0.385 [29]). The shift from fluorite u=0.25 to pyrite u=0.385 is rather large and creates a S-S distance that is clearly a binding one. This is not surprising as in contrast to F- an ion S- is not a closed shell species. It is isoelectronic with a chlorine atom, also undergoing pairing to form Cl2 molecules. Both low spin Fe2+ and the disulfide S22- moeties are closed shell entities, explaining the diamagnetic en semiconducting properties.
The S atoms have bonds with three Fe and one other S atom. The site symmetry at Fe and S positions is accounted for by point symmetry groups C3i and C3, respectively. The missing center of inversion at S lattice sites has important consequences for the crystallographic and physical properties of iron pyrite. These consequences derive from the crystal electric field active at the sulphur lattice site, which causes a polarisation of S ions in the pyrite lattice.[30] The polarisation can be calculated on the basis of higher-order Madelung constants and has to be included in the calculation of the lattice energy by using a generalised Born-Haber cycle. This reflects the fact that the covalent bond in the sulphur pair is ill accounted for in the strictly ionic treatment of Madelung theory.
Arsenopyrite has a related structure with heteroatomic As-S pairs rather than homoatomic ones. Marcasite also possesses homoatomic anion pairs, but the arrangement of the metal and diatomic anions is different than in a pyrite. Despite its name a chalcopyrite does not contain dianion pairs, but single S2- sulfide anions.
Varieties
Cattierite (CoS2) and Vaesite (NiS2) are similar in their structure and belong also to the pyrite group.
Bravoite is a nickel-cobalt bearing variety of pyrite, with >50% substitution of Ni2+ for Fe2+ within pyrite. Bravoite is not a formally recognised mineral, and is named after Peruvian scientist Jose J. Bravo (1874–1928).[31]
Synthetically, materials MX2 with a pyrite structure can be made in the lab with for M a whole series of elements from the Mn column of the d block all the way to the Zn column and for X the chalcogenides S, Se or Te. For most of these systems it is also possible to make continuous solid solution series, so that the Bravo's geological findings are hardly surprising. From a chemical point of view the mineral is therefore likely to vary considerably in composition, depending on which elements were available during its formation.
Distinguishing similar minerals
Chalcopyrite is brighter yellow with a greenish hue when wet and is softer (3.5–4 on Mohs' scale).[32]
Arsenopyrite is silver white and does not become more yellow when wet.
Gallery
The following images show other pyrite forms:
See also
- List of minerals
- Chalcopyrite (Copper Pyrite)
References
- ^ Hurlbut, Cornelius S.; Klein, Cornelis, 1985, Manual of Mineralogy, 20th ed., John Wiley and Sons, New York, pp 285–286, ISBN 0-471-80580-7
- ^ Pyrite on webmineral. Webmineral.com. Retrieved on 2011-05-25.
- ^ Pyrite on. Mindat.org. Retrieved on 2011-05-25.
- ^ Handbook of Mineralogy. (PDF) . Retrieved on 2011-05-25.
- ^ Julia A. Jackson, James Mehl and Klaus Neuendorf, Glossary of Geology, American Geological Institute (2005) p. 82.
- ^ Albert H. Fay, A Glossary of the Mining and Mineral Industry, United States Bureau of Mines (1920) pp. 103–104.
- ^ πυρίτης, Henry George Liddell, Robert Scott, A Greek-English Lexicon, on Perseus
- ^ πύρ, Henry George Liddell, Robert Scott, A Greek-English Lexicon, on Perseus
- ^ James Dwight Dana, Edward Salisbury Dana, Descriptive Mineralogy, 6th Ed., Wiley, New York (1911) p. 86.
- ^ Herbert Clark Hoover and Lou Henry Hoover, translators of Georgius Agricola, [De Re Metallica], The Mining Magazine, London (1912; Dover reprint, 1950); see footnote, p. 112.
- ^ M. E. Fleet and A. Hamid Mumin, Gold-bearing arsenian pyrite and marcasite and arsenopyrite from Carlin Trend gold deposits and laboratory synthesis, American Mineralogist 82 (1997) pp. 182–193
- ^ Andrew Roy, Coal Mining in Iowa, Coal Trade Journal, quoted in History of Lucas County Iowa, State Historical Company, Des Moines (1881) pp. 613–615.
- ^ Zodrow, E (2005). "Colliery and surface hazards through coal-pyrite oxidation (Pennsylvanian Sydney Coalfield, Nova Scotia, Canada)". International Journal of Coal Geology 64: 145. doi:10.1016/j.coal.2005.03.013.
- ^ Oliver Bowles, The Structural and Ornamental Stones of Minnesota, Bulletin 663, United States Geological Survey, Washington (1918) p. 25.
- ^ Tagnithamou, A; Sariccoric, M; Rivard, P (2005). "Internal deterioration of concrete by the oxidation of pyrrhotitic aggregates". Cement and Concrete Research 35: 99. doi:10.1016/j.cemconres.2004.06.030.
- ^ William Angelo, A Material Odor Mystery Over Foul-Smelling Drywall, from the web site of Engineering News Record Construction, dated 1/28/2009.
- ^ Industrial England in the Middle of the Eighteenth Century, Nature, Vol, 83, No. 2113, Thursday, April 28 (1910) pp. 267–268.
- ^ Terkel Rosenqvist (2004). Principles of extractive metallurgy (2nd ed.). Tapir Academic Press. p. 52. ISBN 8251919223. http://books.google.com/?id=I2mg2ine4AEC&pg=PA52&lpg=PA52&dq=pyrite+%22decomposition+temperature%22&q=pyrite%20%22decomposition%20temperature%22.
- ^ K. Ellmer and H. Tributsch (March 11–18, 2000). "Iron Disulfide (Pyrite) as Photovoltaic Material: Problems and Opportunities". Proceedings of the 12th Workshop on Quantum Solar Energy Conversion – (QUANTSOL 2000). http://www.esqsec.unibe.ch/%5Cpub%5Cpub_51.htm.
- ^ The Principles Underlying Radio Communication, Radio Pamphlet No. 40, U.S. Army Signal Corps, Dec. 10 (1918) section 179, pp. 302–305.
- ^ Thomas H. Lee, The Design of Radio Frequency Integrated Circuits, 2nd Ed., Cambridge University Press (2004) pp. 4–6.
- ^ Wadia, Cyrus; Alivisatos, A. Paul; Kammen, Daniel M. (2009). "Materials Availability Expands the Opportunity for Large-Scale Photovoltaics Deployment". Environmental Science & Technology 43 (6): 2072. doi:10.1021/es8019534.
- ^ Cheaper materials could be key to low-cost solar cells by Robert Sanders, 17 February 2009
- ^ Hesse, Rayner W. (2007). Jewelrymaking Through History: An Encyclopedia. Greenwood Publishing Group. p. 15. ISBN 0313335079. http://books.google.com/?id=IVgU0icm948C&pg=PA15.
- ^ Vaughan, D. J.; Craig, J. R. "Mineral Chemistry of Metal Sulfides" Cambridge University Press, Cambridge (1978) ISBN 0-521-21489-0
- ^ W. L. Bragg (1913). "The structure of some crystals as indicated by their diffraction of X-rays". Proc. Roy. Soc. Lond., Ser. A 89 (610): 248–277. Bibcode 1913RSPSA..89..248B. doi:10.1098/rspa.1913.0083. http://www.jstor.org/stable/93488.
- ^ M. Birkholz, S. Fiechter, A. Hartmann, and H. Tributsch (1991). "Sulfur deficiency in iron pyrite (FeS2-x) and its consequences for band structure models". Phys. Rev. B 43 (14): 11926. Bibcode 1991PhRvB..4311926B. doi:10.1103/PhysRevB.43.11926.
- ^ N. E. Brese, and H. G. von Schnering (1994). "Bonding Trends in Pyrites and a Reinvestigation of the Structure of PdAs2, PdSb2, PtSb2 and PtBi2". Z. Anorg. Allg. Chem. 620 (3): 393. doi:10.1002/zaac.19946200302.
- ^ E. D. Stevens, M. L. de Lucia, and P. Coppens (1980). "Experimental observation of the Effect of Crystal Field Splitting on the Electron Density Distribution of Iron Pyrite". Inorg. Chem. 19 (4): 813. doi:10.1021/ic50206a006.
- ^ M. Birkholz (1992). "The crystal energy of pyrite". J. Phys.: Condens. Matt. 4 (29): 6227. Bibcode 1992JPCM....4.6227B. doi:10.1088/0953-8984/4/29/007. http://www.mariobirkholz.de/JPhysC1992.pdf.
- ^ Mindat – bravoite. Mindat.org (2011-05-18). Retrieved on 2011-05-25.
- ^ Pyrite on. Minerals.net (2011-02-23). Retrieved on 2011-05-25.
Further reading
- American Geological Institute, 2003, Dictionary of Mining, Mineral, and Related Terms, 2nd ed., Springer, New York, ISBN 978-3-540-01271-9
- Mineral galleries
External links
- How Minerals Form and Change "Pyrite oxidation under room conditions".